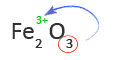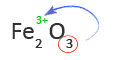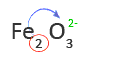Naming chemical compounds is hard at first but you will eventually get the hang of it. You might even become a master of naming chemical compounds!
I will first teach you how to name chemical compounds then I will give you a practice quiz afterwards.
Step 1. Identify Which Type the Compound Belongs
- Binary Acids – hydrogen and a halogen (HF, HCl, HBr and HI only!)
- Ionic Compounds – metal and a nonmetal
- Covalent Compounds – both nonmetals
- HCl is a binary acid
- NaCl is an ionic compound
- CO2 is a covalent compound
Step 2. Name the Compound According to These Steps
Naming Binary Acids
- Write Hydro + root of second element + ic for the first word
- Write acid for the second word
- HCl is Hydrochloric acid
- HBr is Hydrobromic acid
Naming Ionic Compounds
2. Use the subscript of the second element or group of elements as the charge of the first element
Therefore, the first word in the name of Fe2O3 is Iron(III).
5. Use the subscript of the first element as the charge of the second element or group of elements
In this case, the subscript of Fe is not shown. That means the subscript is one and oxygen has a charge of -2 or O2-.
6. Look up the name of the second element or group of elements in a Table of Anions
O2- is oxide
7. Write the name of the anion as the second word
Naming Covalent Compounds
- CO2 is Carbon dioxide
Greek Prefixes